CDSCO- MEDICAL DEVICE IMPORT LICENSE

The Central Drugs Standard Control Organization (CDSCO) is the national regulatory body for medical devices and pharmaceuticals in India. The Medical Device Import License issued by CDSCO is a crucial document required for importing medical devices into India. Here's a description of the process and requirements typically associated with obtaining this license. The Central Drugs Standard Control Organization (CDSCO) plays a pivotal role in regulating medical devices in India. The import of medical devices into the country requires adherence to stringent regulations and obtaining appropriate licenses from the CDSCO. In this comprehensive guide, we will delve into the intricate details of the medical device import license process as governed by the CDSCO.
Introduction to CDSCO

The CDSCO is India's
national regulatory body for pharmaceuticals and medical devices. Established
under the Drugs and Cosmetics Act, 1940, and the Drugs and Cosmetics Rules,
1945, it operates under the Ministry of Health and Family Welfare. Its primary
mandate is to ensure the safety, efficacy, and quality of pharmaceuticals and
medical devices available in the Indian market.
Regulatory Framework For Medical Devices
Medical devices
encompass a wide range of products, including diagnostic equipment, surgical
instruments, implants, and therapeutic devices. Unlike pharmaceuticals, which
undergo rigorous clinical trials before approval, the regulation of medical
devices in India has historically been less stringent. However, recognizing the
importance of ensuring the safety and efficacy of medical devices, the Indian
government has been strengthening its regulatory framework in recent years.
The Medical Device
Rules, 2017, marked a significant shift in the regulation of medical devices in
India. These rules introduced a risk-based classification system for medical
devices, with Class A devices being low-risk and Class D devices being
high-risk. Additionally, the rules mandated that all medical devices must
undergo the process of registration with the CDSCO before being imported, manufactured,
or sold in India.
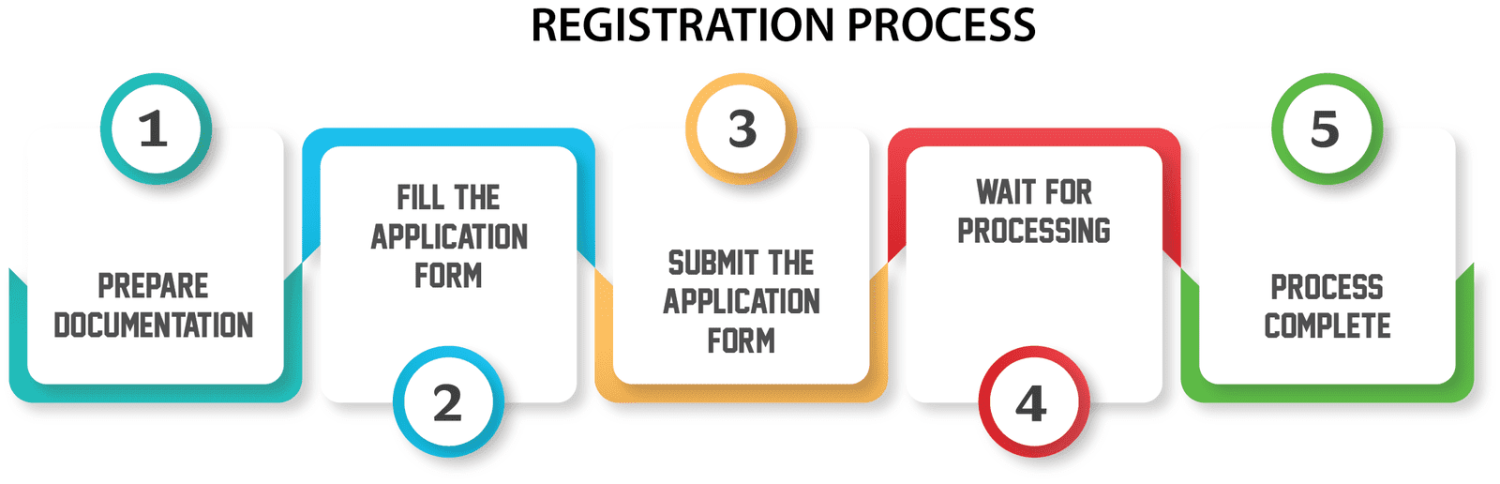
Import License Process
Obtaining a medical
device import license from the CDSCO is a crucial step for companies seeking to
bring medical devices into India. The process involves several stages, each
designed to ensure compliance with regulatory requirements and safeguard public
health.
Preparing the Application
The first step in
obtaining a medical device import license is to prepare a comprehensive
application. This application typically includes detailed information about the
medical device, such as its name, intended use, technical specifications,
manufacturing details, and packaging information. Additionally, applicants must
provide evidence of the device's compliance with relevant quality standards,
such as ISO certification or conformity with Indian Standards (IS).
Application process- To obtain a Medical Device Import License, importers need to submit an
application to the CDSCO. The application form, along with the required
documents, must be submitted electronically through the Sugam online portal,
which is the CDSCO's online platform for regulatory submissions.
Documentation- The application typically requires various
documents, including but not limited to
·
Manufacturer's
information and authorization.
·
Technical
documents related to the medical device, such as specifications, intended use,
labeling, and instructions for use.
·
Regulatory
documents, such as the CE Certificate (if applicable), ISO certification, and
any other relevant certifications or approvals from regulatory authorities in
other countries.
·
Importer's
license.
·
Power of
Attorney (if applicable).
·
Testing
reports and quality control documents.
·
Any other
documents specified by CDSCO.
Quality
Management system- Depending on the class of the medical device, CDSCO may require evidence
of compliance with certain quality management system standards, such as ISO
13485. Importers may need to provide documentation demonstrating conformity to
these standards.
Review Process- CDSCO reviews the application and the submitted
documents to ensure compliance with regulatory requirements. They assess factors
such as the safety, efficacy, and quality of the medical device. The review
process may involve communication between CDSCO and the applicant for
clarifications or additional information.
Approval and
License Issuance- If the
application meets all requirements and the medical device is deemed safe and
effective for use in India, CDSCO grants the import license. The license
specifies the terms and conditions under which the medical device can be
imported and distributed in India.
Validity- The validity period of the Medical Device
Import License may vary depending on factors such as the class of the medical
device and other regulatory considerations. Importers must ensure compliance
with renewal requirements to continue importing the device legally.
Post-Market-Surveillance-
Importers
are typically required to comply with post-market surveillance requirements,
which may include reporting adverse events, conducting recalls if necessary,
and ensuring ongoing compliance with regulatory standards. Even after the import license has been granted,
the regulatory oversight does not end. The CDSCO conducts post-market
surveillance activities to monitor the safety and performance of medical
devices once they are in circulation. This may involve collecting adverse event
reports, conducting product testing, and inspecting distribution channels to
ensure compliance with regulatory requirements.
Conclusion
Obtaining a medical device import license from the CDSCO is a complex and rigorous process that requires careful attention to detail and compliance with regulatory requirements. By adhering to the guidelines outlined in this guide and working closely with regulatory authorities, companies can navigate the import license process successfully and ensure the safety and efficacy of medical devices available in the Indian market. It's essential for importers to thoroughly understand and adhere to CDSCO's requirements to facilitate a smooth import process and ensure compliance with Indian regulatory standards for medical devices.
Recent Posts
Cokion
Cokion Private Limited is an Indian multinational technology company focusing on e-commerce, technology services, online advertising & marketing, headquartered in Bengaluru, Karnataka, India. It has its subsidiary, Cokion Inc., headquartered in Albany, New York, USA.
Welcome to Cokion com., the ultimate online shopping destination tailored for the diverse needs of consumers across the world. At Cokion.com, we pride ourselves on providing a seamless and enjoyable e-commerce experience, offering a wide range of products and services to meet the unique preferences of our customers.
Key Features:
Extensive Product Selection: Discover a vast array of products ranging from electronics and fashion to home goods and more. Cokion.com curates a diverse collection to cater to every aspect of your lifestyle.
User-Friendly Interface: Our intuitive and user-friendly platform ensures a smooth navigation experience. Effortlessly browse through categories, find detailed product information, and enjoy a hassle-free shopping journey.
Secure Transactions: Your security is our priority. Cokion.com employs state-of-the-art encryption and security measures to safeguard your personal information and facilitate secure transactions.
Fast and Reliable Delivery: Enjoy prompt and reliable delivery services across the world. Cokion.com. is committed to ensuring your purchases reach you in a timely manner, enhancing your overall satisfaction.
Responsive Customer Support: Our dedicated customer support team is ready to assist you with any inquiries or concerns. Reach out to us via various channels, and we'll strive to provide swift and effective solutions.
Exclusive Deals and Promotions: Benefit from exciting promotions, discounts, and exclusive deals regularly offered on Cokion.com. Save more while enjoying the quality and convenience of our e-commerce platform.
Mission Statement:
At Cokion.com, our mission is to redefine the online shopping experience for our customers. We aim to become the go-to destination for individuals seeking quality products, exceptional service, and a platform that understands and meets your evolving needs.