Comprehensive Guide to CDSCO Medical Device Registration
Introduction
The Central Drugs Standard Control Organization (CDSCO)
serves as the regulatory authority for medical devices in India. Medical device
registration with the CDSCO is a critical step for manufacturers intending to
market their products in the Indian market. This guide aims to provide a
detailed overview of the medical device registration process, outlining the
requirements, procedures, and key considerations involved.
Overview of CDSCO

Established under the Drugs and Cosmetics Act, 1940, the
CDSCO operates under the purview of the Ministry of Health and Family Welfare.
It is responsible for ensuring the safety, efficacy, and quality of
pharmaceuticals and medical devices in India. With the growing importance of
medical devices in healthcare, the CDSCO has implemented robust regulatory
frameworks to govern their registration and marketing.
Importance
of Medical Device Registration
Medical device registration with the CDSCO is mandatory for
manufacturers intending to market their products in India. It serves as a
regulatory mechanism to ensure that medical devices meet the necessary standards
for safety, efficacy, and quality. Registration also facilitates post-market
surveillance and regulatory oversight to safeguard public health interests.
Regulatory
Framework for Medical Device Registration
The regulatory framework for medical device registration in
India is governed by the Medical Device Rules, 2017. These rules outline the
requirements and procedures for the registration of medical devices with the
CDSCO. The registration process is risk-based, with devices classified into
different classes based on their intended use and potential risks.
Classification
of Medical Devices:
Medical devices are classified into four classes - Class A,
Class B, Class C, and Class D, based on their risk profile. Class A devices are
low-risk, while Class D devices pose the highest risk to patients. The
classification of a device determines the registration requirements and the
level of scrutiny by regulatory authorities.
Key
Requirements for Medical Device Registration:
To obtain registration for a medical device with the CDSCO,
manufacturers must fulfill several key requirements
1.
Technical Documentation: Manufacturers are
required to submit comprehensive technical documentation for their medical
devices, including specifications, design features, manufacturing processes,
and labeling information. This documentation should demonstrate compliance with
relevant quality standards and regulatory requirements.
2.
Clinical Data: For higher-risk devices,
manufacturers may need to provide clinical data demonstrating the safety and
efficacy of the device. This may include clinical trial results, post-market
surveillance data, and evidence of clinical performance.
3.
Quality Management System: Manufacturers must
have a robust quality management system in place to ensure the consistent
quality of their medical devices. Compliance with international quality
standards, such as ISO 13485, is typically required.
4.
Regulatory Approvals: Manufacturers must obtain
any necessary regulatory approvals or certifications from other jurisdictions
where the device is marketed. This may include CE marking for devices sold in
the European Union or FDA approval for devices marketed in the United States.
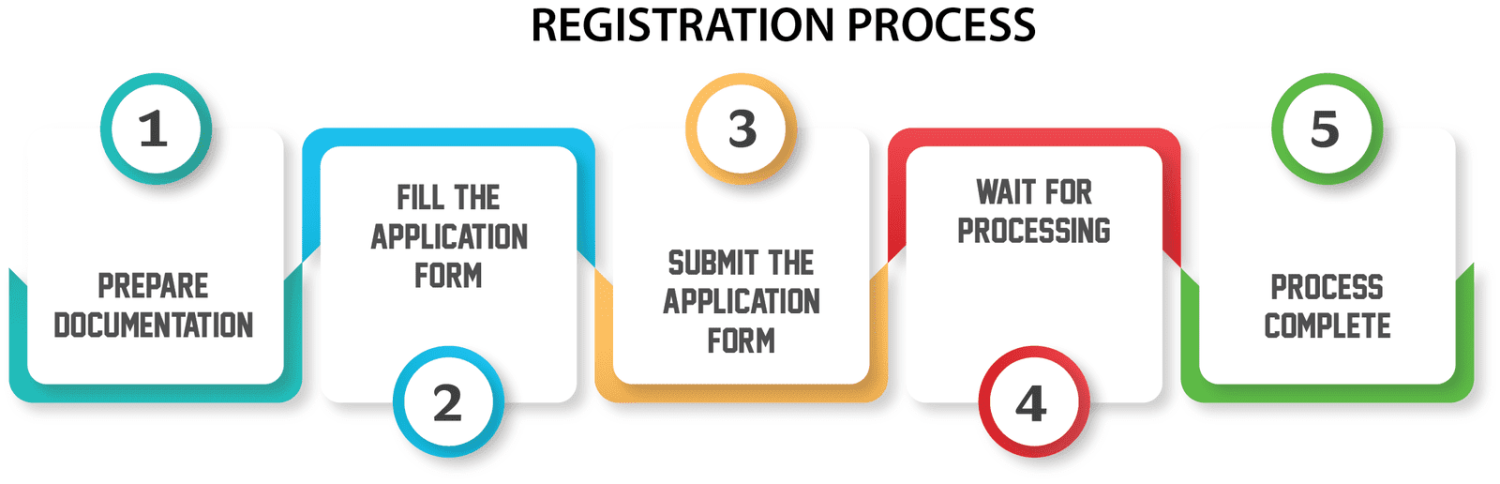
Medical Device Registration Process

The process of registering a medical device with the CDSCO
involves several stages-
1.
Preparing the Application: Manufacturers must
compile all necessary documentation and information required for the
registration process. This includes technical documentation, clinical data,
quality management system certification, and regulatory approvals.
2.
Submission of Application: The completed
application, along with supporting documentation, is submitted to the CDSCO
through the online portal designated for medical device registration.
Manufacturers must ensure that the application is accurate, comprehensive, and
in compliance with regulatory requirements.
3.
Review and Evaluation: The CDSCO conducts a
thorough review of the application and supporting documentation to assess the
safety, efficacy, and quality of the medical device. This may involve technical
assessments, clinical evaluations, and scrutiny of manufacturing processes.
4.
Inspection of Manufacturing Facilities: In some
cases, the CDSCO may conduct on-site inspections of the manufacturing
facilities where the medical device is produced. These inspections aim to
verify compliance with good manufacturing practices (GMP) and other quality
standards.
5.
Granting of Registration: If the CDSCO is
satisfied that the medical device meets all regulatory requirements, it grants
registration allowing the device to be marketed and sold in India. The
registration certificate specifies the conditions and limitations governing the
marketing of the device.
Post-Market
Surveillance and Compliance
Once a medical device is registered and available in the
market, manufacturers are required to comply with post-market surveillance
requirements. This involves monitoring the performance and safety of the device
through adverse event reporting, product testing, and ongoing regulatory compliance.
The CDSCO may conduct periodic inspections and audits to ensure continued
compliance with regulatory standards.
Conclusion
Medical device registration with the CDSCO is a complex and
rigorous process that requires careful attention to detail and compliance with
regulatory requirements. By understanding the regulatory framework and
following the prescribed procedures, manufacturers can navigate the
registration process successfully and ensure that their devices meet the
necessary standards for safety, efficacy, and quality. Ultimately, medical
device registration plays a crucial role in safeguarding public health and
promoting the availability of high-quality medical devices in the Indian market.
Recent Posts
Cokion
Cokion Private Limited is an Indian multinational technology company focusing on e-commerce, technology services, online advertising & marketing, headquartered in Bengaluru, Karnataka, India. It has its subsidiary, Cokion Inc., headquartered in Albany, New York, USA.
Welcome to Cokion com., the ultimate online shopping destination tailored for the diverse needs of consumers across the world. At Cokion.com, we pride ourselves on providing a seamless and enjoyable e-commerce experience, offering a wide range of products and services to meet the unique preferences of our customers.
Key Features:
Extensive Product Selection: Discover a vast array of products ranging from electronics and fashion to home goods and more. Cokion.com curates a diverse collection to cater to every aspect of your lifestyle.
User-Friendly Interface: Our intuitive and user-friendly platform ensures a smooth navigation experience. Effortlessly browse through categories, find detailed product information, and enjoy a hassle-free shopping journey.
Secure Transactions: Your security is our priority. Cokion.com employs state-of-the-art encryption and security measures to safeguard your personal information and facilitate secure transactions.
Fast and Reliable Delivery: Enjoy prompt and reliable delivery services across the world. Cokion.com. is committed to ensuring your purchases reach you in a timely manner, enhancing your overall satisfaction.
Responsive Customer Support: Our dedicated customer support team is ready to assist you with any inquiries or concerns. Reach out to us via various channels, and we'll strive to provide swift and effective solutions.
Exclusive Deals and Promotions: Benefit from exciting promotions, discounts, and exclusive deals regularly offered on Cokion.com. Save more while enjoying the quality and convenience of our e-commerce platform.
Mission Statement:
At Cokion.com, our mission is to redefine the online shopping experience for our customers. We aim to become the go-to destination for individuals seeking quality products, exceptional service, and a platform that understands and meets your evolving needs.









