In Vitro-Diagnostic Device Manufacturing License

Introduction to In Vitro Diagnostic Devices:
In vitro diagnostic devices encompass a wide range of products, including reagents, instruments, and systems used for analyzing biological samples. These devices aid in the detection, diagnosis, and monitoring of diseases such as infectious diseases, cancer, diabetes, cardiovascular disorders, and genetic conditions.
Regulatory Framework:
The manufacturing of IVD devices is subject to stringent regulations imposed by regulatory authorities such as the Food and Drug Administration (FDA) in the United States, the European Medicines Agency (EMA) in the European Union, and other regulatory bodies worldwide. These regulations are designed to ensure the safety, efficacy, and quality of IVD devices throughout their lifecycle, from development and manufacturing to post-market surveillance.
Manufacturing License Requirements:
To manufacture IVD devices, companies must obtain a manufacturing license from the relevant regulatory authority. The requirements for obtaining a manufacturing license vary depending on the jurisdiction and the classification of the device. Typically, the following key requirements must be met:
Quality Management System (QMS): Manufacturers must establish and maintain a robust quality management system compliant with international standards such as ISO 13485:2016. The QMS governs all aspects of manufacturing, including design control, document control, risk management, process validation, and corrective and preventive actions.
Product Classification: IVD devices are classified into different risk classes based on their intended use and potential harm to patients. Manufacturers must determine the appropriate classification of their devices according to regulatory guidelines, which dictate the level of regulatory scrutiny and requirements for approval.
Technical Documentation: Manufacturers must compile comprehensive technical documentation for their IVD devices, including design specifications, manufacturing processes, analytical performance data, clinical evaluation reports, and labeling information. This documentation serves as evidence of compliance with regulatory requirements and is subject to review by regulatory authorities during the licensing process.
Quality Control and Assurance: Manufacturers must implement robust quality control and assurance measures to ensure the consistency, reliability, and safety of their products. This includes establishing specifications for raw materials and components, conducting in-process and finished product testing, and monitoring manufacturing processes for deviations or non-conformities.
Facility Requirements: Manufacturing facilities must meet specific requirements regarding design, construction, equipment, and environmental controls to ensure the integrity of the manufacturing process and the quality of the final product. Facilities may be subject to inspection by regulatory authorities to verify compliance with these requirements.
Adverse Event Reporting: Manufacturers are required to establish procedures for monitoring and reporting adverse events associated with their IVD devices. This includes promptly investigating complaints, documenting adverse events, and reporting serious incidents to regulatory authorities as required by law.
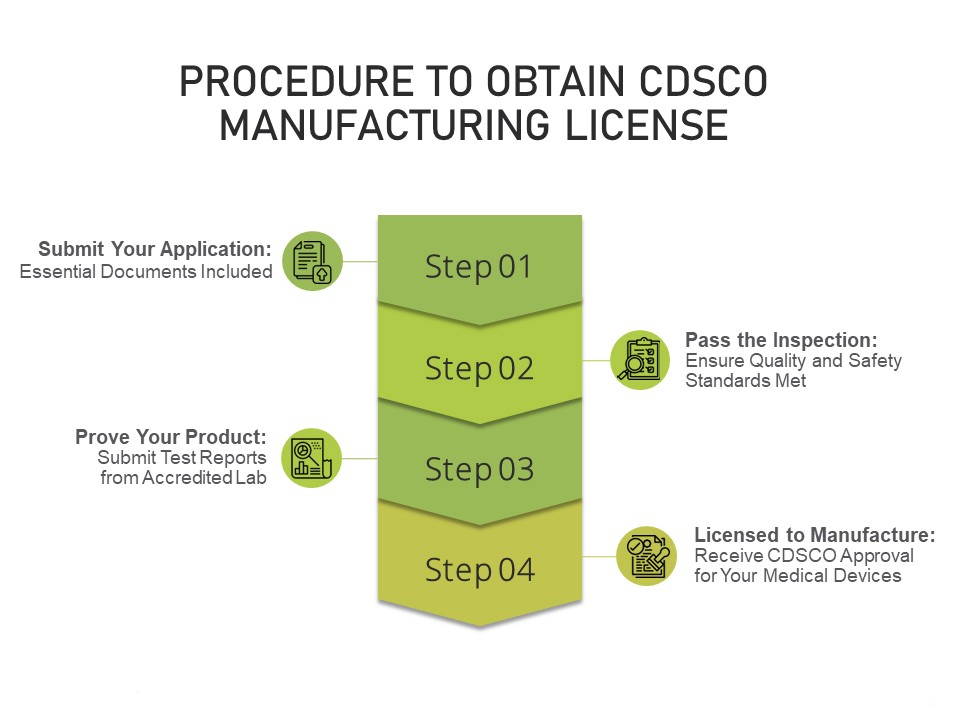
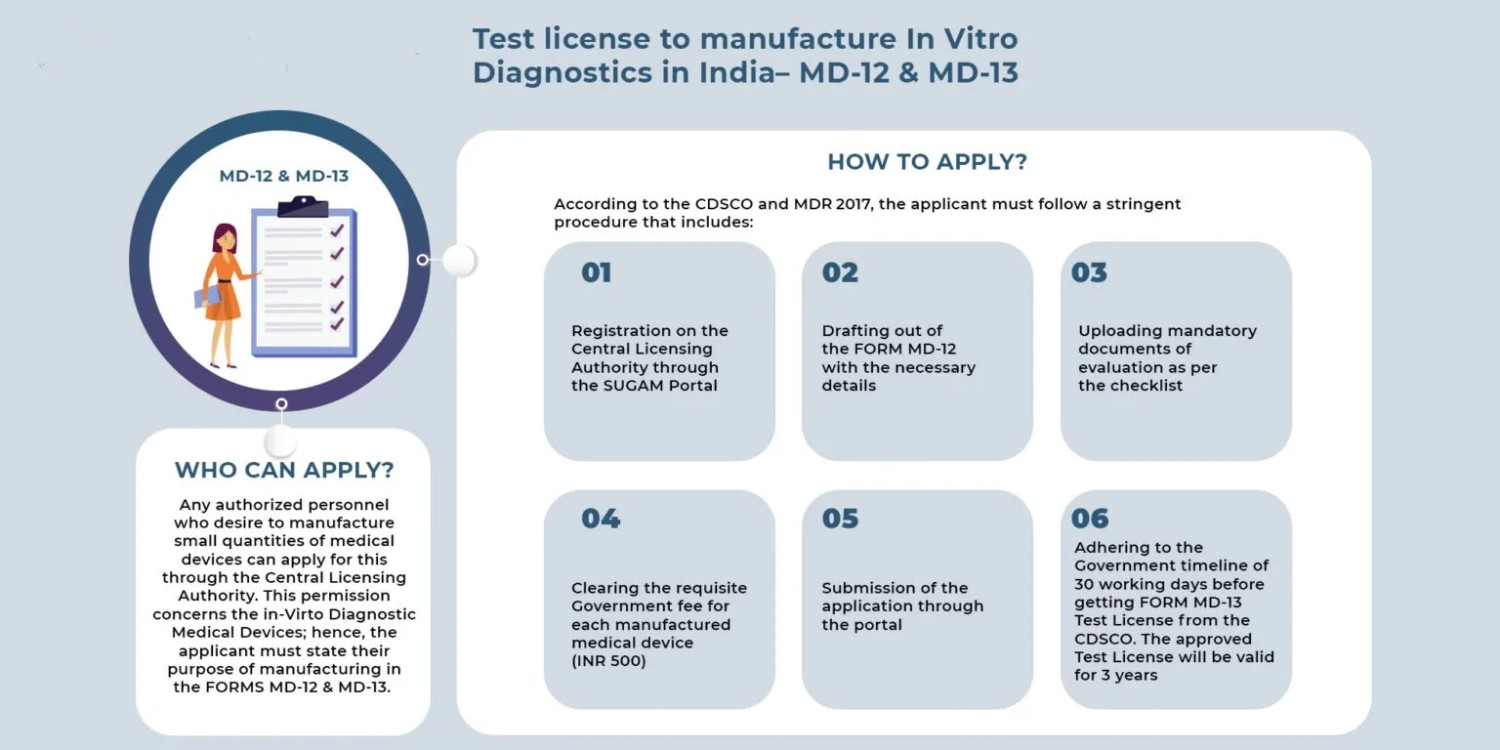
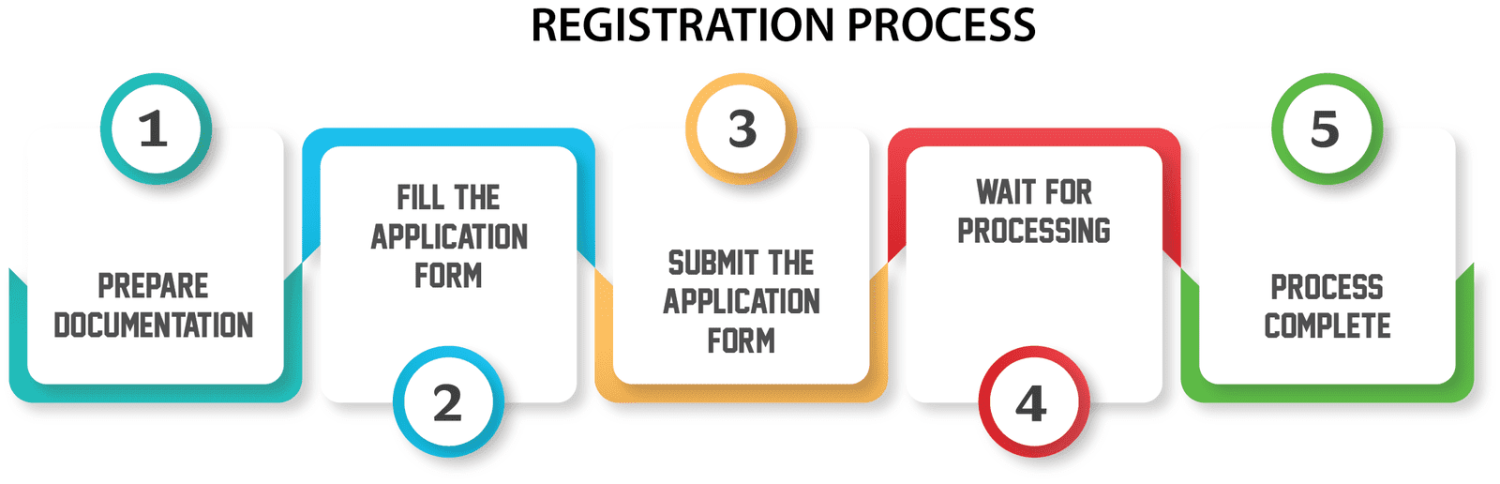
Licensing Process:
The process of obtaining a manufacturing license for IVD devices typically involves several stages, including pre-application activities, submission of the license application, review by regulatory authorities, and post-approval obligations. The following outlines the key steps involved:
Preparation and Planning: Before applying for a manufacturing license, manufacturers must thoroughly understand the regulatory requirements applicable to their IVD devices and ensure that they have the necessary resources, expertise, and infrastructure in place to meet those requirements. This may involve conducting gap assessments, obtaining regulatory guidance, and establishing internal processes and systems.
Application Submission: Manufacturers must prepare and submit a comprehensive license application to the regulatory authority, along with supporting documentation demonstrating compliance with regulatory requirements. The application typically includes details about the company, the manufacturing facility, the IVD device(s), and the quality management system.
Review and Evaluation: Regulatory authorities review the submitted application and supporting documentation to assess the safety, efficacy, and quality of the IVD device(s) and the manufacturing processes. This may involve a thorough review of the technical documentation, facility inspections, and evaluation of clinical data and performance studies.
Communication and Clarification: During the review process, regulatory authorities may request additional information or clarification from the manufacturer to address any concerns or deficiencies identified in the application. Manufacturers must respond promptly and comprehensively to these requests to facilitate the review process.
Decision and Approval: Once the review process is complete, the regulatory authority will issue a decision on the manufacturing license application. If the application is approved, the manufacturer will receive a manufacturing license allowing them to produce and distribute the IVD device(s) in the market. In some cases, the approval may be subject to specific conditions or restrictions.
Post-Approval Obligations: After obtaining a manufacturing license, manufacturers must comply with ongoing regulatory obligations, including post-market surveillance, reporting of adverse events, product labeling updates, and periodic quality audits. Regulatory authorities may conduct inspections and audits of manufacturing facilities to ensure continued compliance with regulatory requirements.
Conclusion:
The manufacturing of in vitro diagnostic devices is a highly regulated process governed by stringent quality and safety standards. Obtaining a manufacturing license for IVD devices requires careful planning, preparation, and compliance with regulatory requirements throughout the lifecycle of the product. By adhering to these requirements and processes, manufacturers can ensure the safety, efficacy, and quality of their IVD devices and contribute to improved patient care and public health outcomes.
For More Information : https://ornatequality.com/
Recent Posts
Cokion
Cokion Private Limited is an Indian multinational technology company focusing on e-commerce, technology services, online advertising & marketing, headquartered in Bengaluru, Karnataka, India. It has its subsidiary, Cokion Inc., headquartered in Albany, New York, USA.
Welcome to Cokion com., the ultimate online shopping destination tailored for the diverse needs of consumers across the world. At Cokion.com, we pride ourselves on providing a seamless and enjoyable e-commerce experience, offering a wide range of products and services to meet the unique preferences of our customers.
Key Features:
Extensive Product Selection: Discover a vast array of products ranging from electronics and fashion to home goods and more. Cokion.com curates a diverse collection to cater to every aspect of your lifestyle.
User-Friendly Interface: Our intuitive and user-friendly platform ensures a smooth navigation experience. Effortlessly browse through categories, find detailed product information, and enjoy a hassle-free shopping journey.
Secure Transactions: Your security is our priority. Cokion.com employs state-of-the-art encryption and security measures to safeguard your personal information and facilitate secure transactions.
Fast and Reliable Delivery: Enjoy prompt and reliable delivery services across the world. Cokion.com. is committed to ensuring your purchases reach you in a timely manner, enhancing your overall satisfaction.
Responsive Customer Support: Our dedicated customer support team is ready to assist you with any inquiries or concerns. Reach out to us via various channels, and we'll strive to provide swift and effective solutions.
Exclusive Deals and Promotions: Benefit from exciting promotions, discounts, and exclusive deals regularly offered on Cokion.com. Save more while enjoying the quality and convenience of our e-commerce platform.
Mission Statement:
At Cokion.com, our mission is to redefine the online shopping experience for our customers. We aim to become the go-to destination for individuals seeking quality products, exceptional service, and a platform that understands and meets your evolving needs.