In vitro-diagnostic device import license

where we aim to shed light on the intricate process of obtaining an import license for in vitro diagnostic (IVD) devices. As the global demand for advanced medical diagnostics continues to rise, ensuring the safe and effective importation of these critical devices is paramount. In this guide, we'll explore the key steps involved in securing an import license for IVD devices, along with important considerations and regulatory requirements to keep in mind.
Understanding In Vitro Diagnostic Devices:
Before delving into the import license process, let's first understand what in vitro diagnostic devices are. These devices are used to perform tests on samples, such as blood, urine, or tissue, outside the body to diagnose diseases or monitor the health status of patients. They play a crucial role in healthcare by providing clinicians with valuable information to guide treatment decisions and improve patient outcomes.
Common examples of IVD devices include blood glucose meters, pregnancy tests, genetic testing kits, and infectious disease diagnostic assays. Given their importance in healthcare delivery, IVD devices are subject to strict regulations to ensure their safety, efficacy, and accuracy.
Key Steps in Obtaining an Import License:

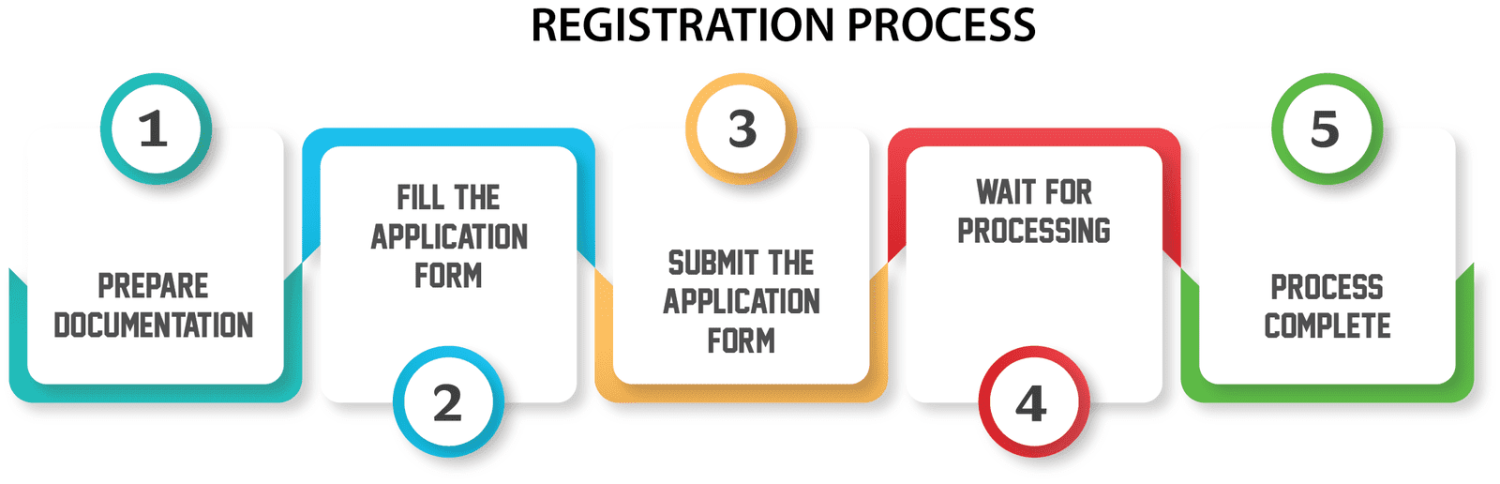
Research Regulatory Requirements: The first step in the import license process is to familiarize yourself with the regulatory requirements governing IVD devices in your target market. Regulations may vary depending on the country or region you're importing into, so it's essential to conduct thorough research and identify the specific requirements that apply to your product.
Determine Classification: IVD devices are typically classified into different risk categories based on factors such as intended use, complexity, and potential harm to patients. Understanding the classification of your device is crucial as it determines the regulatory pathway and requirements for obtaining an import license.
Compile Documentation: Once you've determined the regulatory requirements and classification of your device, you'll need to compile the necessary documentation to support your import license application. This may include product specifications, technical documentation, quality management system certificates, and evidence of compliance with relevant standards and regulations.
Submit Application: With your documentation in hand, you can now submit your import license application to the appropriate regulatory authority. Be sure to follow the application process outlined by the regulatory authority and provide all required information accurately and completely.
Await Approval: Once your application is submitted, you'll need to wait for the regulatory authority to review and assess your application. The timeframe for approval may vary depending on factors such as the complexity of your device and the efficiency of the regulatory authority's review process.
Maintain Compliance: After obtaining your import license, it's essential to maintain compliance with all regulatory requirements throughout the importation process. This includes ensuring that your devices meet quality standards, labeling requirements, and post-market surveillance obligations.
Considerations and Challenges:
While obtaining an import license for IVD devices is essential for market access, it can also be a complex and challenging process. Some common considerations and challenges to keep in mind include:
Regulatory Differences: Regulations governing IVD devices may vary significantly from one country to another, requiring thorough research and understanding of local requirements.
Evolving Regulatory Landscape: Regulatory requirements for IVD devices are subject to change as new technologies emerge and healthcare needs evolve, necessitating ongoing monitoring and adaptation to regulatory changes.
Compliance with Standards: Ensuring compliance with relevant quality standards, such as ISO 13485 for medical devices, is critical for obtaining and maintaining an import license.
Conclusion:
In conclusion, navigating the import license process for in vitro diagnostic devices requires careful planning, attention to detail, and adherence to regulatory requirements. By understanding the regulatory landscape, determining device classification, compiling necessary documentation, and maintaining compliance with standards, you can successfully obtain an import license and bring your IVD devices to market.
we're committed to supporting companies in the medical device industry with regulatory compliance and market access solutions. Contact us today to learn how we can help streamline the import license process for your in vitro diagnostic devices and ensure your products meet regulatory requirements effectively and efficiently.
For More Information: https://ornatecertification.com/
Recent Posts
Cokion
Cokion Private Limited is an Indian multinational technology company focusing on e-commerce, technology services, online advertising & marketing, headquartered in Bengaluru, Karnataka, India. It has its subsidiary, Cokion Inc., headquartered in Albany, New York, USA.
Welcome to Cokion com., the ultimate online shopping destination tailored for the diverse needs of consumers across the world. At Cokion.com, we pride ourselves on providing a seamless and enjoyable e-commerce experience, offering a wide range of products and services to meet the unique preferences of our customers.
Key Features:
Extensive Product Selection: Discover a vast array of products ranging from electronics and fashion to home goods and more. Cokion.com curates a diverse collection to cater to every aspect of your lifestyle.
User-Friendly Interface: Our intuitive and user-friendly platform ensures a smooth navigation experience. Effortlessly browse through categories, find detailed product information, and enjoy a hassle-free shopping journey.
Secure Transactions: Your security is our priority. Cokion.com employs state-of-the-art encryption and security measures to safeguard your personal information and facilitate secure transactions.
Fast and Reliable Delivery: Enjoy prompt and reliable delivery services across the world. Cokion.com. is committed to ensuring your purchases reach you in a timely manner, enhancing your overall satisfaction.
Responsive Customer Support: Our dedicated customer support team is ready to assist you with any inquiries or concerns. Reach out to us via various channels, and we'll strive to provide swift and effective solutions.
Exclusive Deals and Promotions: Benefit from exciting promotions, discounts, and exclusive deals regularly offered on Cokion.com. Save more while enjoying the quality and convenience of our e-commerce platform.
Mission Statement:
At Cokion.com, our mission is to redefine the online shopping experience for our customers. We aim to become the go-to destination for individuals seeking quality products, exceptional service, and a platform that understands and meets your evolving needs.